Hidden role of anion exchange reactions in nucleation of colloidal nanocrystals†
Abstract
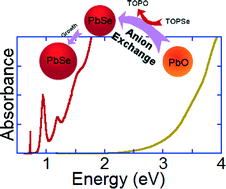
We investigate the processes involved in the nucleation of colloidal lead selenide nanoparticles. Our studies show that an unusual pathway – an anion exchange reaction, causes the nucleation of lead selenide nanocrystals. In this process, one quantum dot is transformed into another due to a substitution of its constituent anions. The existence of this pathway was never anticipated perhaps due to its unusually rapid kinetics. The nucleation and growth kinetics of colloidal lead selenide quantum dots are found to fit well to a two-step process. The rate constant associated with the anion exchange process is found to be four orders of magnitude greater than that of the nanocrystal growth. The complete consumption of the initial oxide nanoparticle thus provides a sharp, temporally well-defined nucleation event.

 Please wait while we load your content...
Please wait while we load your content...