The interplay of thermally activated delayed fluorescence (TADF) and room temperature organic phosphorescence in sterically-constrained donor–acceptor charge-transfer molecules†
Abstract
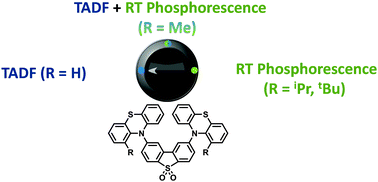
A series of phenothiazine–dibenzothiophene-S,S-dioxide charge-transfer molecules have been synthesized. Increasing steric restriction around the donor–acceptor bond significantly alters contributions from TADF and phosphorescence. Bulky substituents on the 1-(and 9) position(s) of the phenothiazine result in no TADF in the solid state; instead strong phosphorescence is observed at ambient temperature.

 Please wait while we load your content...
Please wait while we load your content...