Umpolung synthesis of branched α-functionalized amines from imines via photocatalytic three-component reductive coupling reactions†
Abstract
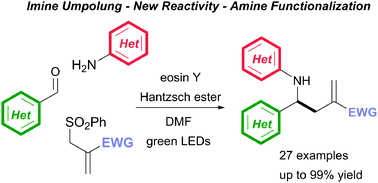
A three component reductive coupling reaction of a (hetero)aromatic amine, a (hetero)aromatic aldehyde and an electron deficient olefin catalysed by eosin Y under green LED light irradiation, for the direct generation of γ-amino acid derivatives, is described. This new umpolung synthesis of amines, which exploits the high nucleophilicity of a putative α-amino radical intermediate, generated via single electron reduction of the in situ generated imine from the Hantzsch ester terminal reductant, is efficient, operationally simple, broad in scope and offers a complementary strategy to existing synthetic approaches.

 Please wait while we load your content...
Please wait while we load your content...