Enzymatic synthesis of natural (+)-aristolochene from a non-natural substrate†
Abstract
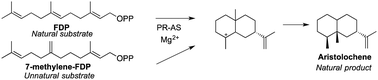
The sesquiterpene cyclase aristolochene synthase from Penicillium roquefortii (PR-AS) has evolved to catalyse with high specificity (92%) the conversion of farnesyl diphosphate (FDP) to the bicyclic hydrocarbon (+)-aristolochene, the natural precursor of several fungal toxins. Here we report that PR-AS converts the unnatural FDP isomer 7-methylene farnesyl diphosphate to (+)-aristolochene via the intermediate 7-methylene germacrene A. Within the confined space of the enzyme's active site, PR-AS stabilises the reactive conformers of germacrene A and 7-methylene germacrene A, respectively, which are protonated by the same active site acid (most likely HOPPi) to yield the shared natural bicyclic intermediate eudesmane cation, from which (+)-aristolochene is then generated.


 Please wait while we load your content...
Please wait while we load your content...