Tetracyclic dihydronaphthalene derivatives via gold-catalyzed aminative homodimerization of ortho-alkynylbenzaldehydes†
Abstract
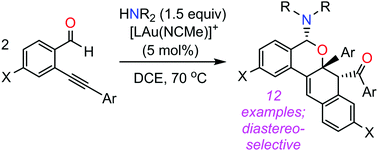
Gold(I) catalyzes domino homodimerization of o-alkynylbenzaldehydes via a sequence of 6-endo-dig-cyclization, amine addition, and [4+2] cycloaddition involving two bicyclic intermediates. The resulting products integrate medicinally relevant 1,2-dihydronaphthalene and isochromene moieties into a single amine-substituted tetracyclic framework.


 Please wait while we load your content...
Please wait while we load your content...