Achieving regio- and stereo-control in the fluorination of aziridines under acidic conditions†
Abstract
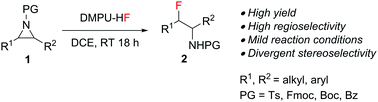
We developed an efficient fluorination protocol that converts easily accessible aziridines into β-fluoroamines, which are important motifs in biologically active molecules. In contrast with traditional fluorination approaches, DMPU–HF has shown both higher reactivity and regioselectivity and good functional group tolerance; thus, a wide scope of β-fluoroamines can now be accessed conveniently. The stereochemical behavior of the ring opening depends on the substitution pattern of the aziridine substrate.


 Please wait while we load your content...
Please wait while we load your content...