Direct synthesis of nitroaryl acetylenes from acetylenes and nitroarenes via oxidative nucleophilic substitution of hydrogen†
Abstract
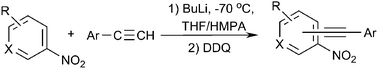
Acetylenic carbanions add to nitroarenes (dinitrobenzenes, nitropyridines, etc.) to form σH-adducts that are subsequently oxidized by DDQ according to the oxidative nucleophilic substitution of hydrogen (ONSH) pathway to give nitroaryl acetylenes.

 Please wait while we load your content...
Please wait while we load your content...