Copper-catalyzed cascade cyclization of 1,5-enynes via consecutive trifluoromethylazidation/diazidation and click reaction: self-assembly of triazole fused isoindolines†
Abstract
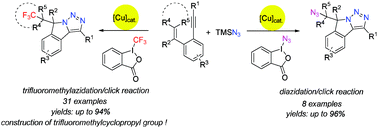
Copper-catalyzed cascade cyclization of 1,5-enynes with two types of hypervalent reagents was developed via consecutive trifluoromethylazidation/diazidation and intramolecular click reaction (CUAAC), providing one-pot self-assembly of triazole fused isoindolines bearing a trifluoromethyl or an azide moiety. Moreover, the construction of a trifluoromethylcyclopropyl unit, which has been considered as a metabolically stable replacement for a tert-butyl moiety and was difficult to access, was also achieved under trifluoromethylazidation conditions. All these reactions exhibited excellent chemoselectivity, good to excellent yields and broad functional group tolerance.

 Please wait while we load your content...
Please wait while we load your content...