Aluminium-mediated aromatic C–F bond activation: regioswitchable construction of benzene-fused triphenylene frameworks†
Abstract
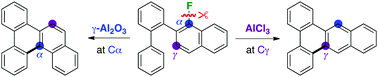
Selective synthesis of benzo[f]tetraphenes or benzo[g]chrysenes was achieved via aromatic C–F bond cleavage and unprecedented regioselective C–C bond formation depending upon the choice of aluminium reagents. On treatment with AlCl3, 2-(biphenyl-2-yl)-1-fluoronaphthalenes afforded exclusively benzo[f]tetraphenes via C–C bond formation on the carbon atom γ to the original position of the fluorine substituent. In contrast, α-selective C–C bond formation was promoted by treatment with γ-Al2O3 to give benzo[g]chrysenes.


 Please wait while we load your content...
Please wait while we load your content...