Traceless directing group mediated branched selective alkenylation of unbiased arenes†
Abstract
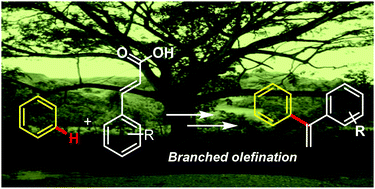
Owing to the synthetic importance of branched olefinated products, we report palladium catalyzed formation of branched olefins facilitated by a C–H activation based protocol. This involves selective insertion of olefins and subsequent decarboxylation using a completely unbiased benzene ring as the starting precursor. The significance of the protocol has been further highlighted by exhibition of functionality tolerance along with a late-stage modification of the branched olefinated products leading to the formation of other functionalized molecules.

 Please wait while we load your content...
Please wait while we load your content...