Copper-catalysed cyanoalkylative cycloetherification of alkenes to 1,3-dihydroisobenzofurans: development and application to the synthesis of citalopram†
Abstract
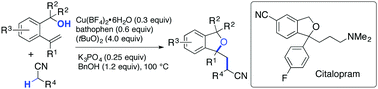
A copper-catalysed cyanoalkylative cycloetherification of alkenes was developed. Heating a solution of substituted (2-vinylphenyl)methanol in MeCN/MeOH (v/v 7/3) in the presence of a catalytic amount of copper(II) tetrafluoroborate hydrate [Cu(BF4)2·6H2O], bathophenanthroline, K3PO4, BnOH and (tBuO)2 afforded 1,3-dihydroisobenzofurans (phthalanes) via formation of one C(sp3)–C(sp3) and one C(sp3)–O bonds. A concise synthesis of citalopram, a marketed anti-depressant drug, was accomplished by applying this novel synthetic transformation.

 Please wait while we load your content...
Please wait while we load your content...