KNH2–KH: a metal amide–hydride solid solution†
Abstract
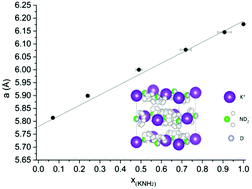
We report for the first time the formation of a metal amide–hydride solid solution. The dissolution of KH into KNH2 leads to an anionic substitution, which decreases the interaction among NH2− ions. The rotational properties of the high temperature polymorphs of KNH2 are thereby retained down to room temperature.


 Please wait while we load your content...
Please wait while we load your content...