Synthesis of photoactivatable azido-acyl caged oxazine fluorophores for live-cell imaging†
Abstract
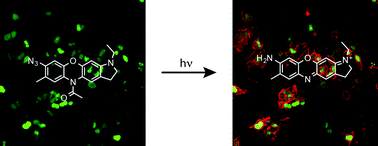
We report the design and synthesis of a photoactivatable azido-acyl oxazine fluorophore. Photoactivation is achieved cleanly and rapidly with UV light, producing a single fluorescent oxazine photoproduct. We demonstrate the utility of azido-acyl caged oxazines for protein specific labeling in living mammalian cells using the TMP-tag technology.

 Please wait while we load your content...
Please wait while we load your content...