Host–guest chemistry that directly targets lysine methylation: synthetic host molecules as alternatives to bio-reagents
Abstract
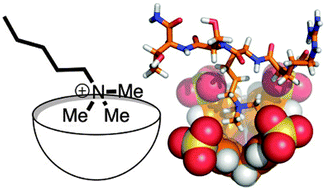
Post-translational methylation is a chemically simple modification, but regulates the function of hundreds of proteins in profound ways. This Feature Article will report on the basic aspects of protein methylation, and will offer a personal perspective on our recent efforts at making supramolecular hosts that can bind and discriminate among post-translationally methylated partners. The article highlights several general lessons drawn from these efforts and related work by other groups. It also describes some ways in which supramolecular approaches are inherently well suited to provide tools that drive new research in the life sciences.
- This article is part of the themed collection: Host–guest chemistry

 Please wait while we load your content...
Please wait while we load your content...