The organocatalytic ring-opening polymerization of N-tosyl aziridines by an N-heterocyclic carbene†
Abstract
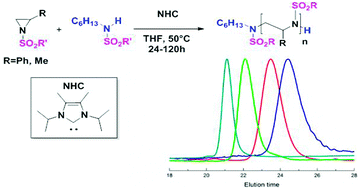
The ring-opening polymerization of N-tosyl aziridines, in the presence of 1,3-bis(isopropyl)-4,5(dimethyl)imidazol-2-ylidene as an organocatalyst and an N-tosyl secondary amine as initiator mimicking the growing chain, provides the first metal-free route to well defined poly(aziridine)s (PAz) and related PAz-based block copolymers.


 Please wait while we load your content...
Please wait while we load your content...