Formation of amides, their intramolecular reactions for the synthesis of N-heterocycles, and preparation of a marketed drug, sildenafil: a comprehensive coverage†
Abstract
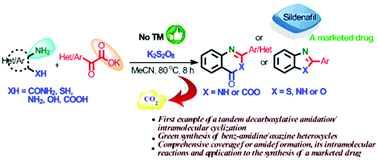
A unified approach to the tandem preparation of diverse nitrogen heterocycles via decarboxylative acylation of ortho-substituted amines with α-oxocarboxylic acids and subsequent intramolecular cyclizations has been developed. The key features of this work include: the first example of transition-metal-free decarboxylative amidation of α-oxocarboxylic acids with ortho-substituted amines, realization of intramolecular cyclization of amides employing nucleophiles that have previously been unexplored, mechanistic investigation of an unprecedented K2S2O8 promoted amide formation and its subsequent intramolecular cyclizations, and application to the synthesis of a best-selling marketed drug.

 Please wait while we load your content...
Please wait while we load your content...