Gold-catalysed cross-coupling between aryldiazonium salts and arylboronic acids: probing the usefulness of photoredox conditions†
Abstract
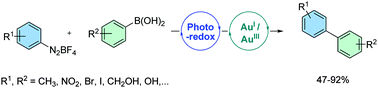
The synthesis of biaryl compounds from aryldiazonium salts and arylboronic acids was achieved using PPh3AuCl as catalyst, CsF as base and acetonitrile as solvent. Combined to photosensitizers, irradiation by blue LEDs allowed accelerating the reaction and expanding its scope. Various functional groups were compatible including bromoaryls, iodoaryls, aldehydes and alcohols. A 2-iodobenzyl alcohol moiety was smoothly introduced by this method, enabling its consecutive isotopic labelling by a Pd-catalysed alkoxycarbonylation.

 Please wait while we load your content...
Please wait while we load your content...