Supersizing pyrrole-modified porphyrins by reversal of the ‘breaking and mending’ strategy†
Abstract
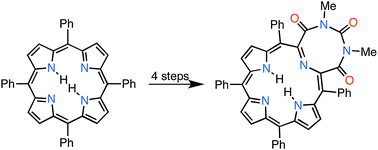
While the ‘breaking and mending of porphyrin strategy’ proved versatile in the generation of a range of pyrrole-modified porphyrins containing 4-, 5-, and 6-membered heterocycles, it failed to access systems incorporating larger rings. A reversal of the strategy – first mending, then breaking – now allowed the formation of a pyrrole-modified porphyrin containing an 8-membered 1,3,6-triazocine-2,4,8-trione heterocycle.

 Please wait while we load your content...
Please wait while we load your content...