Control of the mechanical strength of a bipyridine-based polymeric gel from linear nanofibre to helix with a chiral dopant†
Abstract
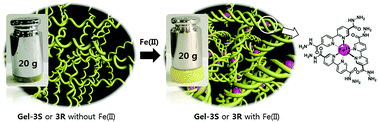
A mixture of building blocks 1 and 2 having hydrazine moieties and aldehyde moieties, respectively, formed a gel by a hydrazone reaction in the absence and presence of cyclohexane diamines as chiral dopants and Fe2+. In particular, the mechanical strength of the helical gel prepared from 1 and 2 in the presence of a chiral dopant and Fe2+ was ca. 10-fold stronger as compared to that of the gel prepared from the building blocks 1 and 2 without a chiral dopant and Fe2+. The improved mechanical strength was attributed to the formation of a helix. The results indicate that the mechanical strength of gels obtained by hydrazone reaction could be controlled by a chiral dopant and Fe2+.

 Please wait while we load your content...
Please wait while we load your content...