Dioxazoles, a new mild nitrene transfer reagent in gold catalysis: highly efficient synthesis of functionalized oxazoles†
Abstract
A gold-catalyzed regioselective [3+2] cycloaddition of ynamides with 1,4,2-dioxazoles was developed and offers a novel approach to obtain highly functionalized oxazoles under mild reaction conditions. 1,4,2-Dioxazole was found to act as an efficient N-acyl nitrene equivalent to trigger a facile generation of α-imino gold–carbene intermediate through the elimination of a ketone.
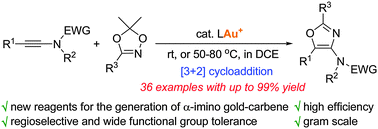

 Please wait while we load your content...
Please wait while we load your content...