Does Fe2+ in olivine-based interstellar grains play any role in the formation of H2? Atomistic insights from DFT periodic simulations†
Abstract
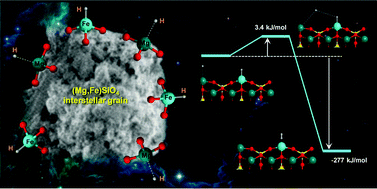
Using periodic DFT-D2 methods, atomistic simulations of interstellar H adsorption and H2 formation on a (010) Fe-containing olivine surface are presented. At variance with the (010) Mg2SiO4 surface and key to these processes are the large Fe/H interaction energies, suggesting that olivine surfaces are good reservoirs of H atoms for subsequent recombination to form H2.


 Please wait while we load your content...
Please wait while we load your content...