Enantioselective intramolecular cyclization of alkynyl esters catalyzed by a chiral Brønsted base†
Abstract
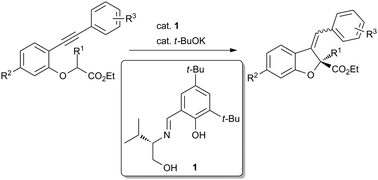
An enantioselective intramolecular cyclization reaction of alkynyl esters was developed, which employs a Brønsted base catalyst generated in situ from a chiral Schiff base and t-BuOK. This reaction is a rare example of the enantioselective intramolecular addition of simple ester enolates to alkynes under Brønsted base catalysis.

 Please wait while we load your content...
Please wait while we load your content...