Visible-light photoredox synthesis of internal alkynes containing quaternary carbons†
Abstract
A novel and efficient visible-light photoredox method for the synthesis of internal alkynes containing quaternary carbons has been developed via coupling reactions of N-phthalimidoyl oxalates of tert-alcohols with 1-(2-(arylsulfonyl)ethynyl)benzenes. The reactions proceeded well at room temperature with good functional group tolerability.
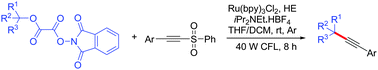

 Please wait while we load your content...
Please wait while we load your content...