Palladium-catalyzed C(sp3)–C(sp2) cross-coupling of homoleptic rare-earth metal trialkyl complexes with aryl bromides: efficient synthesis of functionalized benzyltrimethylsilanes†
Abstract
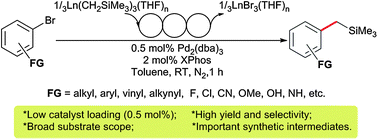
The first C(sp3)–C(sp2) cross-coupling of rare-earth metal alkyl complexes with aryl bromides has been developed. This reaction was conducted at low catalyst loading (0.5 mol%) and exhibited a broad substrate scope, thus providing a facile method for the synthesis of benzyltrimethylsilanes with diverse functional groups.

 Please wait while we load your content...
Please wait while we load your content...