Evidence for an intrinsic binding force between dodecaborate dianions and receptors with hydrophobic binding pockets†
Abstract
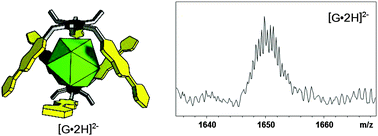
A gas phase binding study revealed strong intrinsic intermolecular interactions between dianionic halogenated closo-dodecaborates [B12X12]2− and several neutral organic receptors. Oxidation of a tetrathiafulvalene host allowed switching between two host–guest binding modes in a supramolecular complex. Complexes of β-cyclodextrin with [B12F12]2− show remarkable stability in the gas phase and were successfully tested as carriers for the delivery of boron clusters into cancer cells.

 Please wait while we load your content...
Please wait while we load your content...