Stabilization of 11/9-helical α/β-peptide foldamers in protic solvents†
Abstract
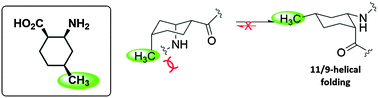
α/β-Peptides with alternating α-amino acid and cis-2-aminocyclohexanecarboxylic acid (cis-ACHC) residues adopt 11/9-helical conformations, the folding propensity of which decreases as the solvent polarity increases. We report a new cis-ACHC analogue, cis-2-amino-cis-4-methylcyclohexanecarboxylic acid, which significantly stabilizes the 11/9-helix propensity in protic solvents.
- This article is part of the themed collection: Foldamers

 Please wait while we load your content...
Please wait while we load your content...