Exploiting redox activity in metal–organic frameworks: concepts, trends and perspectives
Abstract
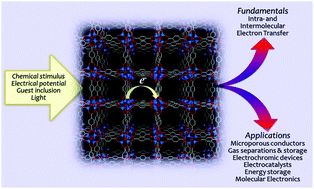
Of the many thousands of new metal–organic frameworks (MOFs) that are now discovered each year, many possess potential redox activity arising from the constituent metal ions and/or organic ligands, or the guest molecules located within their porous structures. Those redox states that can be accessed via postsynthetic redox modulation often possess distinct physical properties; if harnessed, these provide a basis for applications including microporous conductors, electrocatalysts, energy storage devices and electrochemical sensors, amongst others. This feature article highlights the latest developments in experimental, theoretical and computational concepts relevant to redox-active MOFs, including new solid state electrochemical and spectroelectrochemical techniques that have great utility in this field. A particular emphasis is on current and emerging trends at the fundamental level which underscore the importance of this promising class of electroactive materials for a wide range of technologically- and industrially-relevant applications.
- This article is part of the themed collection: 2016 Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...