In situ generated highly active copper oxide catalysts for the oxygen evolution reaction at low overpotential in alkaline solutions†
Abstract
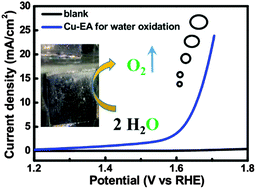
Developing efficient water oxidation catalysts made up of earth-abundant elements has attracted much attention as a step toward for future clean energy production. Herein we report a simple one-step method to generate a low cost copper oxide catalyst film in situ from a copper(II) ethylenediamine complex. The resulting catalyst has excellent activity toward the oxygen evolution reaction in alkaline solutions. A catalytic current density of 1.0 mA cm−2 and 10 mA cm−2 for the catalyst film requires the overpotentials of only ∼370 mV and ∼475 mV in 1.0 M KOH, respectively. This catalytic performance shows that the new catalyst is one of the best Cu-based heterogeneous OER catalysts to date.

 Please wait while we load your content...
Please wait while we load your content...