The Evans Aldol–Prins cyclization: a general and stereoselective method for the synthesis of 2,3,4,5,6-pentasubstituted tetrahydropyrans†
Abstract
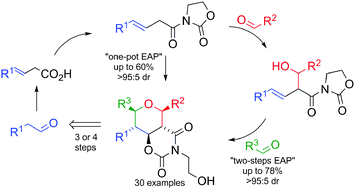
A general and stereoselective method to synthesize 2,3,4,5,6-pentasubstituted tetrahydropyrans in three steps starting from three different aldehydes is described. Key substrates β,γ-unsaturated N-acyloxazolidin-2-ones were subjected to an “Evans Aldol–Prins” protocol to generate five σ-bonds and five stereocenters in only a one-pot process with yields up to 60% and excellent stereoselectivities.

 Please wait while we load your content...
Please wait while we load your content...