On the nature of the stabilisation of the E⋯π pnicogen bond in the SbCl3⋯toluene complex†
Abstract
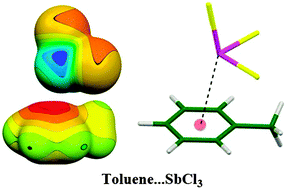
The off-symmetrical structure of the toluene⋯SbCl3 complex is a consequence of the off-centre location of σ-holes at the Sb atom. DFT-SAPT calculations have been used to determine the total interaction energies and their components. The characteristic features of the pnicogen bonding are due to the concert action of electrostatic and dispersion interactions.

 Please wait while we load your content...
Please wait while we load your content...