Iron-catalysed sequential reaction towards α-aminonitriles from secondary amines, primary alcohols and trimethylsilyl cyanide†
Abstract
We have developed a one-pot iron-catalysed sequential reaction of secondary amines with primary alcohols, trimethylsilyl cyanide and TBHP under mild reaction conditions to give the corresponding α-aminonitriles.
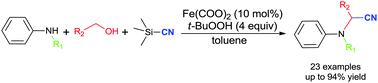

 Please wait while we load your content...
Please wait while we load your content...