Straightforward synthesis of functionalized chroman-4-ones through cascade radical cyclization-coupling of 2-(allyloxy)arylaldehydes†
Abstract
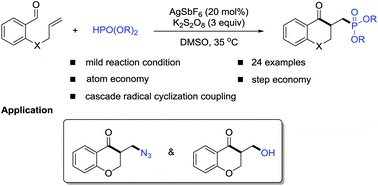
A novel and direct approach to synthesize a series of phosphonate, azide and hydroxy functionalized chroman-4-ones has been developed. The cascade transformation appears to proceed through an intramolecular addition of an in situ generated acyl radical onto the alkene, followed by a selective nucleophilic radical–electrophilic radical cross coupling.

 Please wait while we load your content...
Please wait while we load your content...