Parent and trisubstituted triazacoronenes: synthesis, crystal structure and physicochemical properties†
Abstract
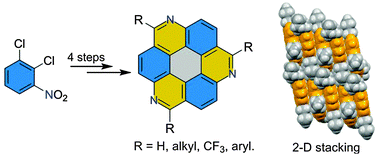
A four-step synthesis of the C3-symmetric parent 1,5,9-triazacoronene (TAC) and its derivatives was achieved using a three-fold Bischler–Napieralski cyclization as the key step. The single-crystal X-ray diffraction of 1b (R = n-Bu) demonstrates that the azacoronene core is perfectly co-planar and the molecules adopt a favorable 2-D “brick-wall” arrangement with strong π–π interactions. The unique stacking, tunable photophysical and electronic properties, and high thermal stability should make them promising candidates for emissive and electron-transport materials.

 Please wait while we load your content...
Please wait while we load your content...