A one-pot amidation of primary nitroalkanes†
Abstract
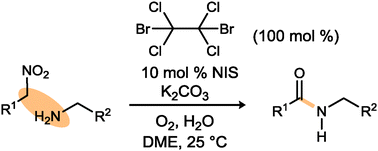
It has been over a half-century since Kornblum demonstrated the conversion of a primary nitroalkane to a carboxylic acid; addition of an amine results in carboxylic acid formation as well. We describe the formation of amides from terminal nitroalkanes in a two-step, one-pot reaction involving tandem halogenation/umpolung amide synthesis (UmAS).

 Please wait while we load your content...
Please wait while we load your content...