Synthesis of geminal bis- and tristriazoles: exploration of unconventional azide chemistry†
Abstract
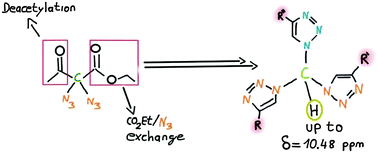
A range of geminal bis- and tristriazoles are presented. These rare and hardly studied compound classes were easily synthesized using ethyl 2,2-diazido-3-oxobutanoate as the common starting point. Firstly, CuAAC-reaction with an alkyne afforded the corresponding deacetylated bistriazoles. Upon further azidation yielding azidomethylenebistriazoles, a second CuAAC-functionalization then led to the creation of the geminal tristriazole compounds.

 Please wait while we load your content...
Please wait while we load your content...