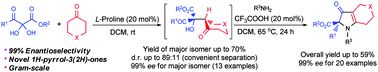
Asymmetric synthesis of 1H-pyrrol-3(2H)-ones from 2,3-diketoesters by combination of aldol condensation with benzilic acid rearrangement†
Abstract
An efficient two-step protocol for the asymmetric synthesis of 1H-pyrrol-3(2H)-one derivatives in 99% ee from conveniently accessed 2,3-diketoesters has been developed.

 Please wait while we load your content...
Please wait while we load your content...