Structural transition and superconductivity in hydrothermally synthesized FeX (X = S, Se)†
Abstract
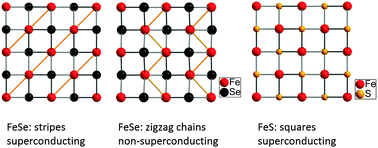
Iron selenide obtained by mild hydrothermal reaction is not superconducting and exhibits a triclinic crystal structure below 60 K unlike superconducting FeSe from conventional solid state synthesis which is orthorhombic. In contrast, tetragonal iron sulphide FeS from hydrothermal synthesis is superconducting but undergoes no structural change on cooling.

 Please wait while we load your content...
Please wait while we load your content...