A highly enantioselective thiolation of sulfonyl indoles to access 3-sec-sulfur-substituted indoles in water†
Abstract
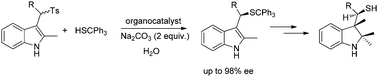
A highly enantioselective approach for the synthesis of 3-alkyl- indole or indoline derivatives with a functional thiol group is presented. The chemistry is based on the asymmetric 1,4-addition of thiol to vinylogous imine intermediates, which are generated in situ from sulfonylindoles. The broad substrate transformation proceeds with high yields (up to 96%) and enantioselectivity (up to 98% ee) in a water-compatible system.

 Please wait while we load your content...
Please wait while we load your content...