Synthesis of glycosylphosphatidylinositol (GPI)-anchor glycolipids bearing unsaturated lipids†
Abstract
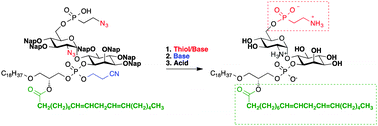
2-Naphthyl-methyl ethers as permanent protecting groups are readily removed under acidic conditions and are key to the synthesis of complex glycosylphosphatidylinositol anchors containing unsaturated lipids. The total synthesis of the GPI pseudo-disaccharide core found on the surface of the Trypanosoma cruzi parasite serves to illustrate the power of the strategy.

 Please wait while we load your content...
Please wait while we load your content...