A rigid lanthanide binding tag to aid NMR studies of a 70 kDa homodimeric coat protein of human norovirus†
Abstract
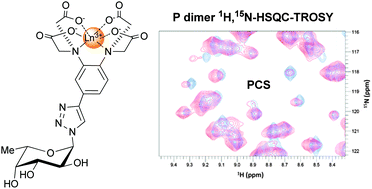
Attachment of human noroviruses to histo blood group antigens is thought to be essential for infection of host cells. Molecular details of the attachment process can be studied in vitro using a variety of NMR experiments. The use of protein NMR based experiments requires assignments of backbone NMR signals. Using uniformly 2H,15N-labeled protruding domains (P-dimers) of a prevalent epidemic human norovirus strain (GII.4 Saga) we have studied the potential of α-L-fucose covalently linked to a rigid lanthanide binding tag to aid backbone assignments using the paramagnetic properties of lanthanide ions. The synthesis of tagged α-L-fucose is reported. Notably, the metal chelating unit connects to the carbohydrate via a triazole linker constructed using click chemistry.

 Please wait while we load your content...
Please wait while we load your content...