The association and aggregation of the metamorphic chemokine lymphotactin with fondaparinux: from nm molecular complexes to μm molecular assemblies†
Abstract
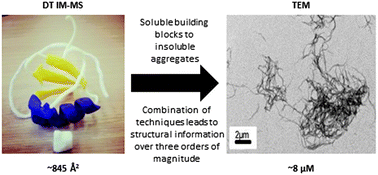
Transmission electron microscopy, mass spectrometry, and drift tube ion mobility-mass spectrometry are used to study the assemblies formed by the metamorphic chemokine lymphotactin in the presence of a model pentameric glycosaminoglycan, fondaparinux. This combination of techniques delineates significant differences in the complexes observed for two forms of the full length protein as well as a truncated form, without the intrinsically disordered C-terminal tail, over a length scale from few nm to μm assemblies.

 Please wait while we load your content...
Please wait while we load your content...