A biphenyl containing two electron-donating and two electron-accepting moieties: a rigid and small donor–acceptor–donor ladder system†
Abstract
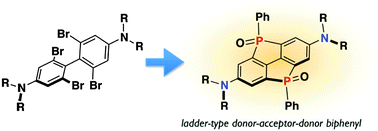
Ladder π-conjugated materials and also push–pull systems belong to important classes of compounds for the development of organic electronic devices. In this communication, a novel π-conjugated material that unifies the properties of both of these classes is presented. The material comprises a rigid biphenyl framework, which bears two bridging electron-accepting phosphine oxide moieties as well as two electron-donating amino groups. The structure and photophysical properties of this compound are discussed and compared with those of a related system lacking the second P-moiety.

 Please wait while we load your content...
Please wait while we load your content...