Dual acid-responsive supramolecular nanoparticles as new anticancer drug delivery systems†
Abstract
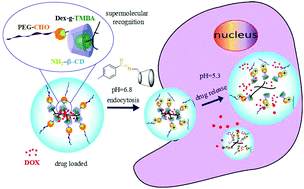
Considering the specific pH gradients of tumour microenvironments, a dual acid-responsive drug delivery system, which can respond to the tumor extracellular and intercellular pH stimuli, has been fabricated via simple host–guest recognition. Firstly, we synthesise 2,4,6-trimethoxybenzaldehyde modified dextran (Dex-TMBA) and mPEG-imine-β-cyclodextrin (PIC), respectively. And then, through the host–guest recognition between the cyclodextrin (CD) of PIC and the benzene ring of Dex-TMBA, a kind of dual acid-responsive supramolecular drug delivery system can be fabricated. Under neutral pH conditions, anticancer drugs can be loaded by forming supramolecular nanoparticles via the host–guest recognition. While, at tumor extracellular pH (∼6.8), the acid-labile benzoic–imine of PIC cleaves and the nanoparticles are amino positively charged to facilitate cell internalization. Subsequently, due to the hydrolysis of acetal bonds in Dex-TMBA under significantly increased acidity in subcellular compartments such as the endosomes (∼5.3), the loaded doxorubicin releases from the endocytosed drug delivery. This dual acid-responsive nanoparticles can efficiently load and release drugs, acting as drug delivery systems for enhancing anticancer efficiency.

 Please wait while we load your content...
Please wait while we load your content...