LC-MS/MS quantification of dimethyl fumarate and methyl hydrogen fumarate in rat blood using tiopronin as trapping reagent
Abstract
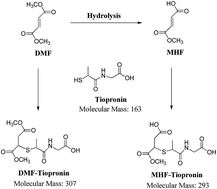
Quantification of dimethyl fumarate (DMF) and its metabolite methyl hydrogen fumarate (MHF) in biological matrices represents a great challenge due to the lack of stability and poor ionization efficiency when using mass spectrometry. Conjugation reactions with endogenous thiols (e.g. glutathione and proteins) and esterase hydrolysis are the main metabolic pathways of DMF in vivo. In the literature, only few analytical methods have been reported for the quantification of DMF and MHF and often non-selective LC-UV detection were utilized. In this investigation, a relatively simple, selective, and sensitive method for the quantification of DMF and MHF in rat blood is described. This method involves the usage of tiopronin as a trapping reagent and sodium fluoride/potassium oxalate as an anti-coagulant to prevent esterase hydrolysis of DMF in blood. Reaction between DMF or MHF and tiopronin was optimized to capture free fractions of DMF and MHF and prevent conjugation reactions with endogenous thiols during sample storage and handling. A protein precipitation technique was employed to extract tiopronin conjugates of DMF and MHF from blood samples. Extracted samples were analyzed using ultra high performance liquid chromatography coupled with tandem mass spectrometry. The method was found to be linear, accurate and precise over the concentration range of 1 to 1000 ng mL−1 for DMF and 10 to 10 000 ng mL−1 for MHF.

 Please wait while we load your content...
Please wait while we load your content...