Assessment of human paraoxonase activity by electrochemistry: a simple and novel approach†
Abstract
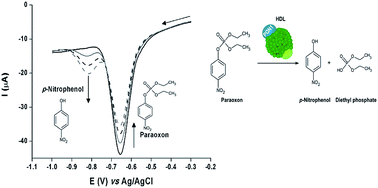
Human serum paraoxonase 1 (EC 3.1.8.1, PON1), a calcium dependent enzyme, is an endogenous free-radical scavenging system with arylesterase, lactonase and paraoxonase activities. The determination of PON1 activity has been gaining an increasing role in clinical diagnosis due to its possible relationship with atherosclerosis and derived diseases. The paraoxonase activity protects against xenobiotic toxicity and was the first to be discovered. It has been the most used activity to assess PON1 status, through the spectrophotometric measurement of the hydrolysis of organophosphate compounds, such as paraoxon. However, these methods are prone to interferences and require specialized equipment. Herein, a simple alternative electrochemical assay for the assessment of PON1 activity is proposed for the first time. The catalytic hydrolysis of paraoxon was monitored by square-wave voltammetry, and the kinetic parameters of Escherichia coli expressed PON1 variant G3C9 (Vappmax 530 ± 40 nM s−1, KappM 1.7 ± 0.4 mM) and native PON1 (Vappmax 390 ± 40 nM s−1, KappM 1.3 ± 0.3 mM), present in human plasma, were determined.

 Please wait while we load your content...
Please wait while we load your content...