Cy5/BHQ dye–quencher pairs in fluorogenic qPCR probes: effects of charge and hydrophobicity†
Abstract
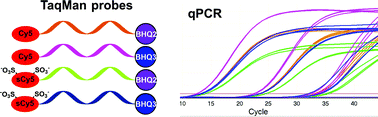
For the first time we used a CuAAC click reaction for the synthesis of cyanine labeled qPCR probes. We performed the comparison of dyes and quenchers (Cy5 vs. disulfo–Cy5 and BHQ2 vs. BHQ3) in several TaqMan probes with different stability of the secondary structure. Based on the studies of thermal quenching of dyes and the increase of fluorescence during probe melting and in the course of qPCR, we suggest that the disulfo–Cy5–BHQ2 pair is a preferable one for molecular beacons due to the highest increase of fluorescence during melting. As the Cy5–BHQ3 pair gave a better Cq and a maximal relative increase of fluorescence in qPCR in comparison to others we recommend this pair for Taqman style probe design.

 Please wait while we load your content...
Please wait while we load your content...