Enantioseparation of fourteen amino alcohols by nonaqueous capillary electrophoresis using lactobionic acid/d-(+)-xylose–boric acid complexes as chiral selectors
Abstract
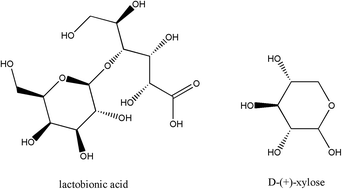
An interesting study on chiral nonaqueous capillary electrophoresis (NACE) was developed in this paper. Two new chiral selectors, a lactobionic acid–boric acid complex and a D-(+)-xylose–boric acid complex were respectively in situ synthesized in nonaqueous background electrolytes (BGEs) containing methanol and triethylamine. They were found to be applicable for the enantioseparation of fourteen amino alcohols including eight β-blockers and six β-agonists by NACE. In order to achieve good enantioseparation, the effects of chiral selector concentration, BGE composition, capillary temperature, and applied voltage were systematically investigated. Under the optimized conditions, most of the tested amino alcohols achieved good chiral resolution. The effects of the molecular structures of chiral selectors and analytes on enantioseparation were discussed in terms of molecular interactions. The method was proved to be suitable for routine analysis of propranolol enantiomers, since it provided satisfactory results during linearity, precision, and accuracy (recovery) studies using the lactobionic acid–boric acid complex as the chiral selector. Good linearity was obtained in the range of 1.0–100.0 μg mL−1 for each propranolol enantiomer and the recoveries for them ranged from 96.4% to 105.9% with the relative standard deviation (RSD) less than 6.4%. This chiral NACE method was applied for the determination of propranolol enantiomers from tablets and the average contents were 101.3% for (R)-propranolol and 98.5% for (S)-propranolol (n = 5).

 Please wait while we load your content...
Please wait while we load your content...