Direct stability characterization of aconite alkaloids in different media by autosampler-mediated incubation-online solid phase extraction-LC-MS/MS†
Abstract
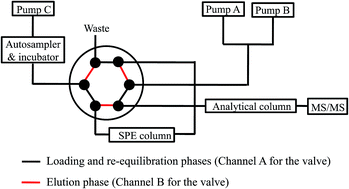
Because degradation might occur at any step of conventional sample preparation protocols, it is of great importance to carry out direct analysis for stability characterization of drug candidates in vitro. Herein, an automated system for directly monitoring the stability of drug candidates in different media was configured by hyphenating autosampler-mediated incubation, online solid phase extraction, and high performance liquid chromatography-tandem mass spectrometry (AMI-SPE-LC-MS/MS). The thermostatic autosampler served as an incubator, and an SPE column was employed to online capture analytes after direct injection of incubated matrices. To achieve sensitive detection, a predictive multiple reaction monitoring approach was introduced to monitor the degradation products. As a proof-of-concept, this system was applied for direct stability characterization of six aconite alkaloids, including aconitine (AC), mesaconitine (MA), hypaconitine (HA), benzoylmesaconine (BMA), benzoylaconine (BAC), and benzoylhypaconine (BHA), which are the primary effective as well as toxic components in aconite-derived herbal medicines. Extensive hydrolysis to generate two degradation products was observed for AC, MA, or HA in phosphate buffer saline (PBS, pH 7.4), PBS fortified with fresh rat plasma and PBS spiked denatured rat plasma, whereas BMA, BAC, and BHA remained intact in PBS, PBS spiked rat plasma, methanol, acetonitrile (ACN), and 50% aqueous ACN. Therefore, care should be taken to maintain the stability of aconite alkaloids, in particular in alkaline media, during metabolic and pharmacokinetic evaluations. More importantly, the developed AMI-SPE-LC-MS/MS system provides a reliable tool for directly and veritably monitoring the in vitro stability of drug candidates.

 Please wait while we load your content...
Please wait while we load your content...