Trapping chlorine radicals via substituting nitro radicals in the gas phase†
Abstract
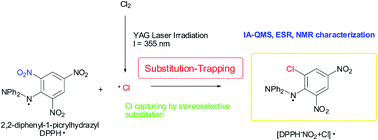
Although chlorine radicals are strong atmospheric oxidants, their direct spectroscopic detection in the environment (gas phase) using conventional analytical techniques has not been reported. Herein, chlorine radicals (Cl˙), generated by the YAG laser photolysis (λ = 355 nm) of Cl2 in the gas phase, were captured by using 2,2-diphenyl-1-picrylhydrazyl (DPPH˙). The product was identified as DPPH, wherein NO2 was substituted by Cl˙ at the ortho-carbon on the picryl aromatic ring, and was characterized using ion attachment ionization-quadrupole mass spectrometry. This reaction mechanism is a rare example of the detection and characterization of radical species, where radical spin densities on trapping reagents and frontier orbital interactions play important roles.

 Please wait while we load your content...
Please wait while we load your content...