Design of a molecular imprinting biosensor with multi-scale roughness for detection across a broad spectrum of biomolecules†
Abstract
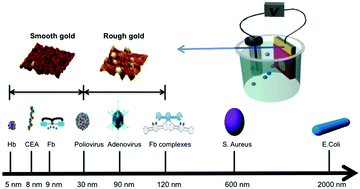
The molecular imprinting technique has tremendous applications in artificial enzymes, bioseparation, and sensor devices. In this study, a novel molecular imprinting (MI) biosensor platform was developed for the detection of a broad range of biomolecules with different sizes. Previously this method has been applied to 2D molecular imprinting, where the height of the self-assembled monolayer (SAM) of around 2 nm limited the maximum dimensions of the molecule that can be imprinted to create template-shaped cavities. In order to match the size of the imprinted molecules with the height of the SAM, we propose a model for 3D molecular imprinting where the analyte is sequestered within a niche created by the surface roughness. The SAM is assembled on the walls of the niche, forming a 3D pattern of the analyte uniquely molded to its contour. Surfaces with multi-scale roughness were prepared by evaporation of gold onto electropolished (smooth) and unpolished (rough) Si wafers, where the native roughness was found to have a normal distribution centered around 5 and 90 nm respectively. Our studies using molecules with size ranging on a nanometer scale, from proteins of a few nanometers to bacteria of hundreds of nanometers, showed that when the size of the analyte matched the roughness range of the gold surface, the molecular imprinting process was optimized for the best biosensing performance. After optimization, the MI biosensor platform enabled the identification and quantification of a broad range of biomolecules with great discrimination abilities. Hemoglobin under different pH values and several mutated fibrinogen molecules can also be well differentiated through the test.

 Please wait while we load your content...
Please wait while we load your content...